Getting screened for cervical cancer in the United States may soon become much more convenient. Women might no longer need to endure uncomfortable in-office procedures involving stirrups, speculums, or even take time off work to visit a doctor.
Instead, they’ll soon have the option to collect their own vaginal samples at home.
The US Food and Drug Administration has approved the first self-collection device for at-home cervical cancer screening, called the Teal Wand, according to the women’s health company Teal Health. The device received the FDA’s “breakthrough device” designation last year, expediting the review process.
Available by prescription, the Teal Wand will be included in Teal Health’s at-home self-collection kit. Users will collect their own samples, which are then mailed to a lab for testing for human papillomavirus (HPV)—the leading cause of cervical cancer. Early detection of HPV can help identify women at risk for developing the disease.
Cervical cancer screening is typically performed by gynecologists who collect samples for either an HPV test, a Pap smear (also known as cervical cytology), or both. The Pap test involves examining cervical cells for abnormalities that may indicate precancerous or cancerous changes, while the HPV test detects the presence of high-risk HPV types known to cause cervical cancer.
Last year, the FDA approved similar self-collection cervical cancer screening kits for use in clinical settings, such as doctor’s offices, urgent care centers, and mobile clinics. At that time, two healthcare companies—biotech firm Roche and medical technology company Becton, Dickinson and Company—announced that the FDA had authorized the use of self-collected samples with their respective HPV tests.
Now, Teal Health’s at-home kit takes that a step further by allowing patients to collect their own sample from the comfort of home using the Teal Wand, which is then analyzed using Roche’s HPV testing system, according to Teal Health CEO Kara Egan. The key distinction is that the Teal Wand is approved specifically for at-home use, eliminating the need for travel or in-person appointments.
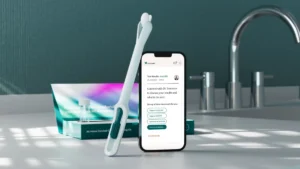
“You can comfortably do it from home,” Egan explained. “You simply request a kit on Teal’s website, consult with a provider who prescribes the kit, then privately collect your sample at home and mail it to a lab, where it’s tested using the Roche Cobas HPV platform.”
“The results are reviewed by a clinician and then shared with the patient,” said Kara Egan. “If the results come back positive, a healthcare provider will follow up and guide you through any necessary next steps.”
According to clinical trial data from Teal Health, using the Teal Wand for self-collection is just as accurate for cervical cancer screening as a sample collected by a healthcare professional, Egan noted.
“It’s the same test with the same level of accuracy—but now you can do it comfortably at home,” she said. “It offers women more choices, and with the growth of telehealth, it’s another way to improve access to care.”
Teal Health plans to begin shipping its at-home self-collection kits in June, starting in California, with a nationwide rollout to follow. Interested individuals can join the waitlist through the company’s website.
Teal Health is currently in discussions with health insurance providers to have the at-home self-collection kit covered, according to CEO Kara Egan. For those without insurance, pricing details for the kit are expected to be announced within the next month.
The American Cancer Society welcomed the FDA’s recent approval.
“Despite the proven benefits of cervical cancer screening, many eligible individuals are not screened regularly,” said Dr. William Dahut, the society’s chief scientific officer, in an email on Friday. “Most cervical cancer cases occur in people who have either never been screened or haven’t been screened in a long time. That’s why the FDA’s approval of the first at-home cervical cancer screening test is such a significant milestone—it adds a new option that could help save lives.”
Cervical Cancer Screening: Why It Matters
According to the U.S. Centers for Disease Control and Prevention (CDC), women can lower their risk of cervical cancer by getting the HPV vaccine, avoiding smoking, using condoms, undergoing regular screening, and consulting a doctor if test results are abnormal.
Data from 2021 shows that roughly 1 in 4 adults are not up to date with recommended cervical cancer screenings.
“Many women feel anxious about undergoing a traditional Pap smear or find the procedure uncomfortable, which often leads them to delay this critical screening,” said Dr. Ami Vaidya, co-chief of gynecologic oncology at Hackensack University Medical Center’s John Theurer Cancer Center, in a statement on Friday.
The newly approved at-home screening device could become a powerful tool to help increase screening rates, particularly for women without regular access to healthcare.
“Any test that aids in the early detection of cervical cancer is a win,” Vaidya said.
Current Screening Guidelines
The U.S. Preventive Services Task Force (USPSTF) recommends:
- Women ages 21 to 29: Pap test (cervical cytology) every three years
- Women ages 30 to 65:
- Pap test every three years, or
- High-risk HPV testing alone every five years, or
- Co-testing with both HPV testing and Pap test every five years
It’s estimated that around 80% of people will contract an HPV infection at some point in their lives. HPV, or human papillomavirus, is a group of over 150 related viruses primarily transmitted through sexual contact. The virus includes both low-risk strains—commonly linked to warts—and high-risk strains, which have been associated with several cancers, including cervical, anal, penile, and oropharyngeal cancers.
In most cases, HPV infections resolve on their own within two years. However, if the virus persists, it can lead to serious health issues, including cancer.
Cervical cancer screening remains vital because early-stage cervical cancer often presents no symptoms. As the disease progresses, symptoms such as abnormal vaginal bleeding or unusual discharge may appear. Treatment options for cervical cancer typically include surgery, chemotherapy, and radiation therapy.